Experimental and computational characterization of photosensitized conformational effects mediated by protoporphyrin ligands on human serum albumin†
Abstract
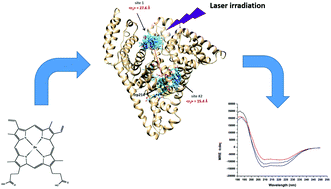
When investigating the interaction between proteins and protoporphyrins in aqueous solution, one typically has to contend with the tendency of the latter to form polydispersed aggregates. The interference of aggregated protoporphyrins manifests, at least, at two levels: aggregates sequester the majority of the protoporphyrin molecules in solution and prevent their interaction with the proteins, but also their presence interferes with optical experiments such as absorption and fluorescence spectroscopy. In this study we present a protocol which uses dialysis and centrifugation to eliminate the aggregates and yield solutions dominated by non-covalent complexes of albumin (HSA) and protoporphyrins. The elimination of the aggregates enabled us to observe effects which had not been previously observed such as eliminating the discrepancy between the binding constants obtained through the quenching of HSA fluorescence and the one obtained through the emission of the protoporphyrins. Moreover the elimination of the aggregated protoporphyrins enabled us to reveal the occurrence of fluorescence resonance energy transfer (FRET) between the Trp214 residue of HSA and the porphyrin ligands. FRET data were then used to estimate the location of metal free as well as Zn-protoporphyrin IX relative to the well-known location of Trp214. This information was used to refine docking simulations to find the best binding site for the two protoporphyrins. In addition we observed that the irradiation of the protoporphyrins in the visible region prompts small conformational changes in HSA that appear to be largely due to tertiary modifications.


 Please wait while we load your content...
Please wait while we load your content...