Formal total synthesis of salvianolic acid N†
Abstract
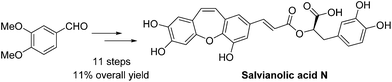
An efficient synthetic pathway for the total synthesis of salvianolic acid N has been reported. The key reaction steps, the Wittig reaction for Z-stereoselectivity and an intramolecular cyclization for a seven membered ring skeleton, have been optimized to improve the synthetic feasibility and provide the best conditions in terms of yield. Moreover, a notable reaction is the reaction of the deprotected allylic group with Pd catalyst. An improved overall yield of 11% has been achieved for salvianolic acid N starting from 3,4-dimethoxybenzaldehyde in 11 steps.


 Please wait while we load your content...
Please wait while we load your content...