Structural revision of two unusual rhamnofolane diterpenes, curcusones I and J, by means of DFT calculations of NMR shifts and coupling constants†
Abstract
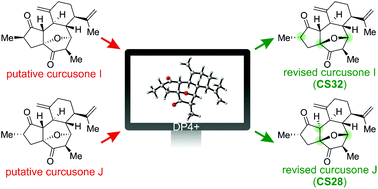
The stereochemical revision of two recently reported rhamnofolane diterpenes, curcusones I and J, was enabled by quantum calculations of NMR shifts and coupling constants at DFT levels. DP4+ results and reexamination of the NMR data suggest that curcusones I and J should be revised as CS32 and CS28, respectively.


 Please wait while we load your content...
Please wait while we load your content...