Highly efficient asymmetric construction of novel indolines and tetrahydroquinoline derivatives via aza-Barbier/C–N coupling reaction†
Abstract
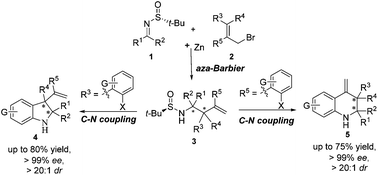
Highly stereoselective syntheses of chiral indolines and tetrahydroquinolines are achieved by combining the asymmetric Zn-mediated allylation of chiral N-tert-butanesulfinyl imines with efficient intramolecular C–N cross-coupling. Herein, the advantages of such a synthetic strategy are illustrated by the synthesis of indolines and tetrahydroquinolines with quaternary stereocenters and multi-substituted 1-oxo-1,2,3,4-tetrahydroisoquinolines.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...