Synthesis of trifluoroalkyl or difluoroalkyl phenanthridine derivatives via cascade reaction using an intramolecular cyano group as a radical acceptor under photoredox catalysis†
Abstract
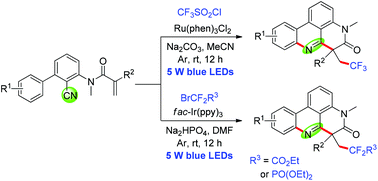
In the presence of Ru(phen)3Cl2 or fac-Ir(ppy)3 under visible-light irradiation, the addition of fluorinated radicals to N-arylacrylamides followed by an intramolecular cyano group insertion cascade cyclization process produced trifluoroalkyl or difluoroalkyl phenanthridine derivatives in moderate to good yields. Three easily available fluoroalkylated reagents CF3SO2Cl, BrCF2CO2Et and BrCF2PO(OEt)2 were used as the sources of fluorinated radicals.


 Please wait while we load your content...
Please wait while we load your content...