Hydrogen-free reductive amination using iron pentacarbonyl as a reducing agent†
Abstract
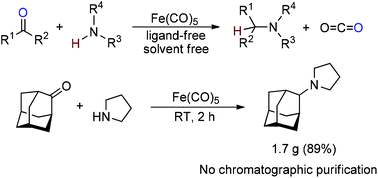
We developed solvent-free reductive amination without an external hydrogen source using iron pentacarbonyl as a reducing agent. Neither a catalyst nor any other additives were employed. Various types of substrates are suitable for the reaction, including those with low reactivity, e.g. benzophenone. Among others, the protocol tolerates bromo-, cyano-, benzyloxy-, pyrimidyl and styryl moieties.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...