Pd-Catalyzed regioselective intramolecular dehydrogenative C-5 cross coupling in an N-substituted pyrrole-azole system†
Abstract
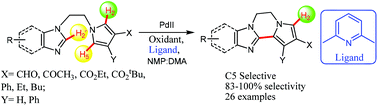
Functionalized polycyclic pyrrole-azole structures possessing fused six membered and seven membered rings were directly synthesized via ligand-enabled, Pd-catalyzed, site selective, intramolecular cross couplings of N-substituted pyrrole-azoles. C5-H activation in the presence of a reactive C2-H remains a challenge that needs to be addressed and this was targeted to be resolved through the present approach by specifically generating the cyclized products with 83–100% selectivity. The featured methodology provides a novel disconnection for the synthesis of pyrrole containing alkaloids and medicinal compounds.


 Please wait while we load your content...
Please wait while we load your content...