Total synthesis of selaginpulvilins A and C†
Abstract
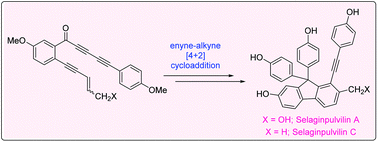
An efficient formal total synthesis of two compounds from the selaginpulvilin family of natural products, selaginpulvilin A and C, has been successfully achieved. The tetradehydro Diels–Alder (TDDA) reaction between an enyne and alkyne has been utilized for the creation of the necessary fluorene skeleton. Attempts at the conversion of selaginpulvilin A to selaginpulvilin B, F and H were unsuccessful.


 Please wait while we load your content...
Please wait while we load your content...