Efficient cross-coupling of aryl/alkenyl triflates with acyclic secondary alkylboronic acids†
Abstract
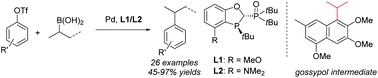
Aryl–secondary alkyl cross-coupling with aryl sulfonate esters as coupling partners remains a significant challenge. Efficient cross-coupling between aryl/alkenyl triflates and acyclic secondary alkylboronic acids is realized for the first time to provide a series of sterically congested acyclic secondary alkyl arenes/olefins in good to excellent yields. The employment of sterically bulky P,P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ligand L1/L2 is crucial for the high yields and selectivities. The method has enabled a concise and 4-step synthesis of a key intermediate of male contraceptive agent and PAF antagonist gossypol.
O ligand L1/L2 is crucial for the high yields and selectivities. The method has enabled a concise and 4-step synthesis of a key intermediate of male contraceptive agent and PAF antagonist gossypol.


 Please wait while we load your content...
Please wait while we load your content...