Improved synthesis of symmetrically & asymmetrically N-substituted pyridinophane derivatives†
Abstract
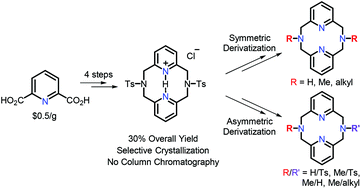
The N,N′-di(toluenesulfonyl)-2,11-diaza[3,3](2,6)pyridinophane (TsN4) precursor was sought after as a starting point for the preparation of various symmetric and asymmetric pyridinophane-derived ligands. Various procedures to synthesize TsN4 had been published, but the crucial problem had been the purification of TsN4 from the larger 18- and 24-membered azamacrocycles. Most commonly, column chromatography or other laborious methods have been utilized for this separation, yet we have found an alternate selective dissolution method upon protonation which allows for multi-gram scale output of TsN4·HCl. This optimized synthesis of TsN4 also led to the development of symmetric RN4 derivatives as well as the asymmetric derivative N-(tosyl)-2,11-diaza[3,3](2,6)pyridinophane (TsHN4). Using this TsHN4 precursor, different N-substituents can be added to create a library of asymmetric RR′N4 macrocyclic ligands. These asymmetric RR′N4 derivatives expand the utility of the RN4 framework in coordination chemistry and the ability to study the electronic, steric, and denticity effects of these pyridinophane ligands on the metal center.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...