α-Trifluoromethylated tertiary homoallylic amines: diastereoselective synthesis and conversion into β-aminoesters, γ- and δ-aminoalcohols, azetidines and pyrrolidines†
Abstract
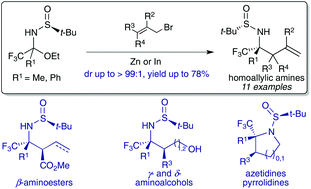
The diastereoselective addition of allyl zinc and allylindium derivatives to α-trifluoromethyl N-tert-butanesulfinyl hemiaminals, bench stable precursors of aryl and alkyl trifluoromethyl ketimines, allows the synthesis of homoallylic amines containing a tetrasubstituted carbon stereocentre bearing a trifluoromethyl group with good diastereoselectivities (up to dr > 99 : 1). This approach was also suitable for accessing chiral homoallylic amines bearing two contiguous stereocenters. The synthetic usefulness of N-tert-butanesulfinyl homoallylamines was illustrated by preparing various trifluoromethylated nitrogen containing bifunctional synthons (aminoesters, aminoalcohols) and small azaheterocycles (azetidines, pyrrolidines).


 Please wait while we load your content...
Please wait while we load your content...