Total syntheses of all tri-oxygenated 16-phytoprostane classes via a common precursor constructed by oxidative cyclization and alkyl–alkyl coupling reactions as the key steps†
Abstract
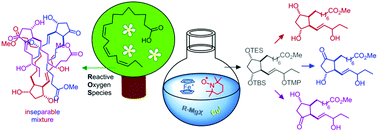
A unified strategy for the total synthesis of the methyl esters of all phytoprostane (PhytoP) classes bearing two ring-oxygen atoms based on an orthogonally protected common precursor is described. Racemic 16-F1t-, 16-E1-PhytoP and their C-16 epimers, which also occur as racemates in Nature, were successfully obtained. The first total synthesis of very sensitive 16-D1t-PhytoP succeeded, however, it quickly isomerized to more stable, but so far also unknown Δ13-16-D1t-PhytoP, which may serve as a more reliable biomarker for D-type PhytoP. The dioxygenated cyclopentane ring carrying the ω-chain with the oxygen functionality in the 16-position was approached by a radical oxidative cyclization mediated by ferrocenium hexafluorophosphate and TEMPO. The α-chain was introduced by a new copper-catalyzed alkyl–alkyl coupling of a 6-heptenyl Grignard reagent with a functionalized cyclopentylmethyl triflate.


 Please wait while we load your content...
Please wait while we load your content...