Structure–reactivity correlations of the abnormal Beckmann reaction of dihydrolevoglucosenone oxime†
Abstract
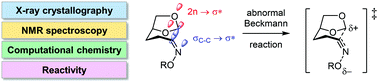
A structural, spectroscopic and computational study of a series of oximes was undertaken to investigate how geometric and structural changes relevant to the reaction coordinate for the Beckmann reaction (normal Beckmann) and Beckmann fragmentation (abnormal Beckmann) manifest in the ground state. X-ray structures of a range of oximes derived from dihydrolevoglucosan (Cyrene™; which undergoes the abnormal Beckmann reaction), in which the oxygen substituent was systematically varied were determined. As the electron demand of the OR group increased, the major structural changes included lengthening of the N–OR bond distance, and a decrease in the magnitude of the C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O bond angle, consistent with the changes seen for cyclohexanone oximes, which undergo the normal Beckmann reaction. However, unique to the Cyrene oximes, an increase in the length of the fissile C1–C2 bond was observed, which correlated with a decrease in the 13C–13C 1-bond coupling constants as the electron demand of the OR substituent increased. Computational studies of Cyrene and cyclohexanone oximes using Natural Bond Orbital analysis support an electronic structure involving n(O) → σ*C1–C2 and σC1–C2 → σ*N–O localized orbital interactions.
N–O bond angle, consistent with the changes seen for cyclohexanone oximes, which undergo the normal Beckmann reaction. However, unique to the Cyrene oximes, an increase in the length of the fissile C1–C2 bond was observed, which correlated with a decrease in the 13C–13C 1-bond coupling constants as the electron demand of the OR substituent increased. Computational studies of Cyrene and cyclohexanone oximes using Natural Bond Orbital analysis support an electronic structure involving n(O) → σ*C1–C2 and σC1–C2 → σ*N–O localized orbital interactions.


 Please wait while we load your content...
Please wait while we load your content...