Facile access to functionalized indenes and fused quinolines by regioselective 5-enolexo-dig Michael addition and cyclization reactions†
Abstract
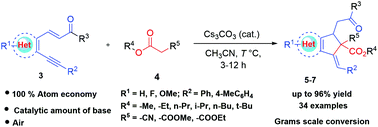
Herein we report a facile approach to synthesise multi-substituted indenes and cyclopenta[b]quinolines under mild conditions. The reaction proceeds via Michael addition between commercially available cyanoacetate/malonic esters and α,β-unsaturated ketones. The synthetic methodology involves enolate mediated regio- and stereoselective intramolecular 5-enolexo-dig cyclization promoted by a catalytic base. The products stereoselectively form cis-isomers for indenes and trans-isomers for cyclopenta[b]quinolines, albeit with the presence of steric hindrance at a quaternary carbon substituted by active methylene compounds. The reaction pathway was investigated by isolating the reaction intermediate. This synthetic transformation was achieved with various aromatic and heteroaromatic Michael acceptors and the desired products were obtained in high to excellent yields. The reaction is scalable up to gram level with only 10 mol% of base.


 Please wait while we load your content...
Please wait while we load your content...