Chemoselective N-arylation of aminobenzamides via copper catalysed Chan–Evans–Lam reactions†
Abstract
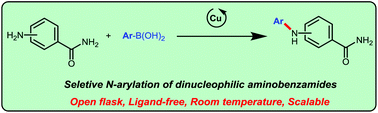
Chemoselective N-arylation of unprotected aminobenzamides was achieved via Cu-catalysed Chan–Evans–Lam cross-coupling with aryl boronic acids for the first time. Simple copper catalysts enable the selective arylation of amino groups in ortho/meta/para-aminobenzamides under open-flask conditions. The reactions were scalable and compatible with a wide range of functional groups.


 Please wait while we load your content...
Please wait while we load your content...