Pd-Catalyzed C–H aziridination of 3,3,5,5-tetrasubstituted piperazin-2-ones†
Abstract
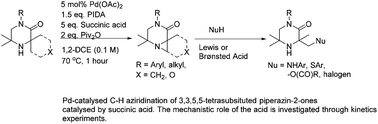
A palladium mediated C–H aziridination reaction of 3,3,5,5-substituted-piperazin-2-ones has been developed using phenyliodonium diacetate (PIDA) and succinic acid to give synthetically useful bicyclic aziridines, in moderate to good yields. Succinic acid was found to be key for selectively promoting C–N bond formation (aziridination) and suppressing competitive acetoxylation. Analysis of the reaction kinetics revealed the role of succinic acid in promoting an equilibrium between monomeric and dimeric palladium species in the rate determining step of the reaction. The aziridines can be ring-opened by nucleophiles under Lewis or Brønsted acidic conditions to give formal C–H functionalized products. The reaction conditions can be further manipulated to produce acetoxylated, diacetoxylated and even triacetoxylated materials through the use of acetic acid and increased oxidant stoichiometry.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...