Substituent effects on stereoselectivity of dihalocarbene reactions with cyclohexadiene and on the reactivity of bis-dihalocyclopropanes in electrophilic nitrations en route to pyrimidine N-oxides†
Abstract
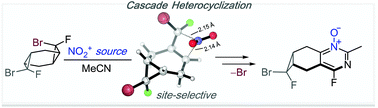
Tricyclic bis-adducts of cyclohexa-1,4-diene with bromofluorocarbene and non-symmetric adducts with both bromofluoro- and dichlorocarbenes were synthesised selectively. The treatment of the bis-adducts with nitrating reagents in acetonitrile affords the products of heterocyclization of a sole dihalogenocyclopropane into 4-fluoropyrimidine N-oxide. The difference in the reactivity of bis-cyclopropanes with different sets of halogen substituents leads to selective heterocyclization of bromofluorocyclopropanes without affecting the dichlorocyclopropane moiety.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...