The enantioselective addition of 1-fluoro-1-nitro(phenylsulfonyl)methane to isatin-derived ketimines†
Abstract
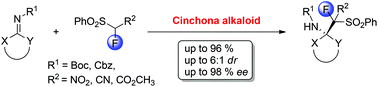
An asymmetric organocatalytic addition of fluorinated phenylsulfonylnitromethane to isatin-derived ketimines was developed. The reaction was efficiently catalyzed by a chiral tertiary amine, cinchonine. This methodology provides a new type of optically active compound with two adjacent quaternary carbon stereocenters in good yield (up to 96%), with moderate diastereoselectivity (up to 5.7 : 1 dr) and excellent enantioselectivity (up to 98/96% ee).


 Please wait while we load your content...
Please wait while we load your content...