An asymmetric acetate-Mannich reaction of chiral isatin derived ketimines and its applications†
Abstract
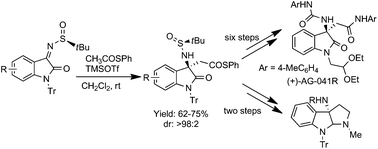
A highly efficient TMSOTf-mediated asymmetric acetate-Mannich reaction of isatin derived tert-butylsulfinyl ketimines and S-phenyl thioacetate was developed to afford the direct synthesis of indole-based β3,3-amino acid thioester with excellent selectivity (dr > 98 : 2). Syntheses of (+)-AG-041R and 3-aminopyrroloindoline have been accomplished utilizing the developed method.


 Please wait while we load your content...
Please wait while we load your content...