Highly regioselective gold-catalyzed formal hydration of propargylic gem-difluorides†
Abstract
Herein, we report a highly regioselective gold-catalyzed formal hydration of propargylic gem-difluorides. Not only does this transformation provide access to versatile fluorinated building blocks that were difficult or hardly possible to access beforehand, but it also represents a rare case of a highly regioselective gold-catalyzed hydroalkoxylation of internal alkynes and puts forward the utility of the difluoromethylene unit as a directing group in catalysis.
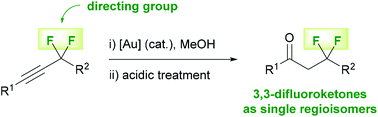


 Please wait while we load your content...
Please wait while we load your content...