A modular approach towards functionalized highly stable self-complementary quadruple hydrogen bonded systems†
Abstract
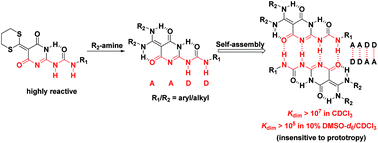
Self-complementary quadruple hydrogen bonded systems have shown potential as key building blocks for developing various supramolecular polymers. Opportunities for the introduction of multiple functionalities would further augment, in principle, their application potential. Herein, we report a novel modular approach to simultaneously introduce two closely aligned side chains into AADD-type self-complementary quadruple hydrogen-bonding systems. Dithiane-tethered ureidopyrimidinone has been used as a reactive intermediate to efficiently attach closely aligned side chains by simply reacting with amines to form highly stable molecular duplexes. These duplexes featuring AADD-type arrays of hydrogen bonding codes are highly stable in non-polar solvents (Kdim > 1.9 × 107 M−1 in CDCl3) as well as in polar solvents (Kdim > 105 in 10% DMSO-d6/CDCl3). Another notable feature of these self-assembling systems is their insensitivity to prototropy-related issues owing to their prototropic degeneracy, which will enhance their application potential in supramolecular chemistry.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...