Asymmetric synthesis of dihydrocoumarins via the organocatalytic hetero-Diels–Alder reaction of ortho-quinone methides†
Abstract
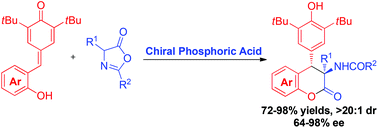
Enantioselective organocatalytic inverse-electron-demand hetero-Diels–Alder reactions of in situ generated ortho-quinone methides with azlactones have been developed. This strategy could generate various chiral dihydrocoumarins bearing a quaternary amino acid moiety in high yields (up to 94%) and stereoselectivities (up to 99 : 1 e.r., >20 : 1 dr) in the presence of a chiral phosphoric acid catalyst. The useful transformation and mechanistic study of this process are also presented.


 Please wait while we load your content...
Please wait while we load your content...