Decoding glycan protein interactions by a new class of asymmetric N-glycans†
Abstract
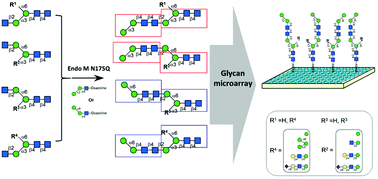
N-Glycans are normally involved in crucial physiological and disease processes by interactions with glycan-binding proteins. So far structurally defined N-glycans have been good candidates for glycan binding study. Herein, a class of homogeneous asymmetric N-glycans was synthesized by coupling glycan-oxazoline and N-glycans using EndoM N175Q catalyzed quick glycan extension. Branch-biased binding and spacial inhibition caused by the bulky group on the other branch of N-glycan were observed in glycan protein interactions involving lectins and these glycans by glycan microarray study. These new compounds are valuable for functional glycomic studies to better understand new functions of glycans and glycan-binding proteins.


 Please wait while we load your content...
Please wait while we load your content...