Synthesis of monoalkylidene diketopiperazines and application to the synthesis of barettin†
Abstract
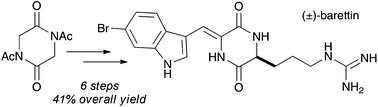
The synthesis of barettin, a selective serotonin receptor inhibitor and potent antibiofouling natural product, is described. The synthesis starts with the diketopiperazine nucleus intact and the side chains are installed using iterative aldol condensations. The route represents a general strategy for synthesis of a wide array of mono-alkylidene diketopiperazine structures, including those derived from non-canonical amino acid residues.


 Please wait while we load your content...
Please wait while we load your content...