Stereoselective synthesis of 17,18-epoxy derivative of EPA and stereoisomers of isoleukotoxin diol by ring opening of TMS-substituted epoxide with dimsyl sodium†
Abstract
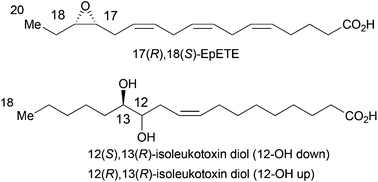
The reaction of TMS-substituted epoxy alcohols (and derivatives) with dimsyl sodium (NaDMSO) to give 1-alkene-3,4-diols was used for the synthesis of enantiomerically enriched 17(R),18(S)-EpETE and two diastereoisomers of isoleukotoxin diol. In the synthesis of 17(R),18(S)-EpETE, the α-ethoxyethyl ether (EE) of the epoxy alcohol derived from (R)-1-TMS-1-penten-3-ol underwent reaction with NaDMSO to give the mono EE-protected 1-hexene-3,4-diol. The aldehyde obtained by hydroboration/oxidation was subjected to Wittig reaction to afford a mono EE-protected diol. The corresponding mono mesylate was prepared and subjected to epoxide ring formation to afford 17(R),18(S)-EpETE stereoselectively. Similarly, a reaction of the anti epoxy alcohol derived from (R)-1-TMS-1-octen-3-ol with NaDMSO gave the anti form of 1-nonene-3,4-diol, which was converted to 12(S),13(R)-isoleukotoxin diol through Wittig reaction. 12(R),13(R)-Isoleukotoxin diol was synthesized in a similar manner.


 Please wait while we load your content...
Please wait while we load your content...