N-Acylsuccinimides: twist-controlled, acyl-transfer reagents in Suzuki–Miyaura cross-coupling by N–C amide bond activation†
Abstract
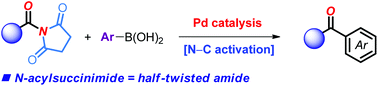
The palladium-catalyzed Suzuki–Miyaura cross-coupling of N-acylsuccinimides as versatile acyl-transfer reagents via selective amide N–C bond cleavage is reported. The method is user-friendly since it employs commercially-available, air-stable reagents and catalysts. The cross-coupling is enabled by half-twist of the amide bond in N-acylsuccinimides. These highly effective, crystalline acyl-transfer reagents present major advantages over perpendicularly twisted N-acylglutarimides, including low price of the succinimide activating ring, selective metal insertion under redox neutral conditions and high stability of the amide bond towards reaction conditions. Mechanistic studies indicate that oxidative addition is the rate limiting step in this widely applicable protocol.


 Please wait while we load your content...
Please wait while we load your content...