Pd-Catalysed Suzuki coupling of α-bromoethenylphosphonates with organotrifluoroborates: a general protocol for the synthesis of terminal α-substituted vinylphosphonates†
Abstract
A general and robust protocol for the synthesis of terminal α-substituted vinylphosphonates via Suzuki coupling of α-bromovinylphosphonates with organotrifluoroborates has been successfully developed. This method features a broad substrate scope, great functional group compatibilities, and easy scale-up ability. In addition to easy access of nucleophiles, a straightforward synthesis of electrophiles was also realized with diethyl α-bromoethenylphosphonate as the starting material. With a combination of Pd2(dba)3/SPhos as the catalyst, a range of α-alkyl, aryl, heteroaryl, and alkynyl substituted ethenylphosphonates could be nicely accessed under mild conditions. As a synthetic application, the terminal vinylphosphonate was utilized as an effective Michael acceptor in the visible-light-promoted Giese reaction.
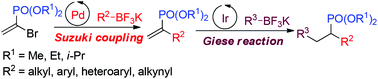


 Please wait while we load your content...
Please wait while we load your content...