Synthesis of functionalized imidazolidine-2-thiones via NHC/base-promoted aza-benzoin/aza-acetalization domino reactions†
Abstract
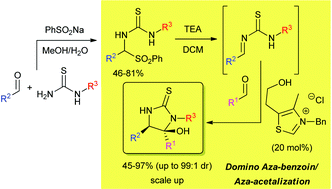
A strategy for the synthesis of biologically relevant 5-hydroxy-imidazolidine-2-thione derivatives is presented. A novel class of α-sulfonylamines have been suitably prepared (46–81% yield) as precursors of formal benzylidenethiourea acceptors; these are generated in situ and intercepted by N-heterocyclic carbene (NHC)-activated aldehydes affording open-chain aza-benzoin-type adducts, which in turn undergo an intramolecular aza-acetalization reaction in a one-pot fashion. A thiazolium salt/triethylamine couple proved to be the more effective system to trigger the domino sequence giving the target heterocycles in good yields (45–97%) and diastereoselectivities (up to 99 : 1 dr). The multigram scale synthesis and elaboration of a selected 5-hydroxy-imidazolidine-2-thione compound is also described.


 Please wait while we load your content...
Please wait while we load your content...