A diversity oriented synthesis of natural product inspired molecular libraries†
Abstract
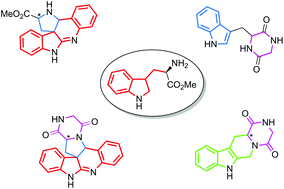
Natural products are the source of innumerable pharmaceutical drug candidates and also form an important aspect of herbal remedies. They are also a source of various bioactive compounds. Herein we have leveraged the structural attributes of several natural products in building a library of architecturally diverse chiral molecules by harnessing R-tryptophan as the chiral auxiliary. It is converted to its corresponding methyl ester 1 which in turn provided a bevy of 1-aryl-tetrahydro-β-carbolines 2a–d, which were then converted to chiral compounds via a diversity oriented synthetic strategy (DOS). In general, intermolecular and intramolecular ring rearrangements facilitated the formation of the final compounds. Four different classes of molecules with distinct architectures were generated, adding up to nearly twenty-two individual molecules. Phenotypic screening of a representative section of the library revealed two molecules that selectively inhibit MCF7 breast cancer cells with IC50 of ∼5 μg mL−1 potency.


 Please wait while we load your content...
Please wait while we load your content...