Azetidine-derived dinuclear zinc catalyzed asymmetric phospha-Michael addition of dialkyl phosphite to α,β-unsaturated carbonyl compounds†
Abstract
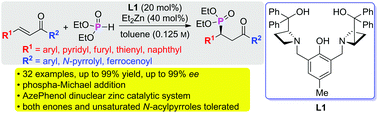
The asymmetric phospha-Michael addition of dialkyl phosphite to α,β-unsaturated carbonyl compounds by using an azetidine-derived dinuclear zinc catalyst was described. The catalyst was proved to be general and efficient for a broad spectrum of enones and α,β-unsaturated N-acylpyrroles. A series of phosphonate-containing compounds were generated with excellent enantioselectivities (up to 99% ee) and chemical yields (up to 99%) under mild conditions without using additives. The products were obtained with more than 95% ee for 23 examples of α,β-unsaturated carbonyl compounds. A positive nonlinear effect was observed and the possible mechanism was proposed.


 Please wait while we load your content...
Please wait while we load your content...