Silver-catalyzed decarboxylative acylation of quinoxalin-2(1H)-ones with α-oxo-carboxylic acids†
Abstract
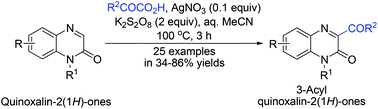
A novel silver-catalyzed decarboxylative acylation of α-oxo-carboxylic acids was developed, by which various 3-acyl quinoxalin-2(1H)-ones were synthesized by direct C–H bond acylation of quinoxalin-2(1H)-ones. In this method, α-oxo-carboxylic acids served as efficient acylating reagents to in situ generate the required active acyl radical. Its excellent chemoselectivity allowed the molecular diversity of 3-acyl quinoxalin-2(1H)-ones to be achieved by convenient functionalizations of both N1- and C3-positions.


 Please wait while we load your content...
Please wait while we load your content...