Ionic-surfactant-coated subtilisin: activity, enantioselectivity, and application to dynamic kinetic resolution of secondary alcohols†
Abstract
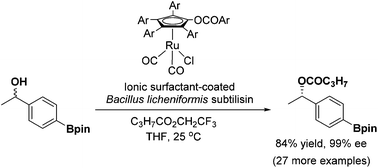
In this work, we explored the utility of ionic-surfactant-coated Bacillus licheniformis subtilisin (ISCBLS) as the catalyst for the dynamic kinetic resolution of secondary alcohols. ISCBLS was prepared by freeze-drying Bacillus licheniformis subtilisin with both ionic surfactant 1 and dextrin. ISCBCL displayed 9300-fold enhanced activity relative to its native counterpart in the transesterificaion of N-acetyl phenylalanine ethyl ester with 1-propanol in hexane and 12 800-fold enhanced activity in the transesterification of trifluoroethyl butyrate with 1-phenylethanol in THF. The enantioselectivity of ISCBLS was examined with 50 secondary alcohols as the substrates for kinetic resolution in the presence of trifluoroethyl butyrate. ISCBLS displayed synthetically useful enantioselectivity for most of the secondary alcohols tested. The enantioselectivity of ISCBLS was in particular good to high for m- or p-substituted 1-phenylethanols. The DKRs of these secondary alcohols by the combination of ISCBLS and a ruthenium-based racemization catalyst provided the products of (S)-configuration with good results (80–94% yield, 90–99% ee). It is concluded that ISCBLS is of great use as the enantiocomplementary counterpart of (R)-selective lipase for the dynamic kinetic resolution of secondary alcohols.


 Please wait while we load your content...
Please wait while we load your content...