Synthesis and biological evaluation of novel teixobactin analogues†
Abstract
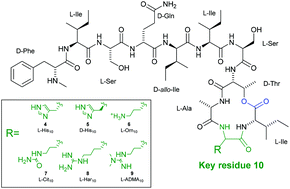
The cyclic depsipeptide, teixobactin, possesses promising activity against a range of antimicrobial-resistant (AMR) pathogenic bacteria, including Staphylococcus aureus and Mycobacterium tuberculosis. Teixobactin contains a number of non-canonical residues, including the synthetically challenging amino acid, L-allo-enduracididine, complicating clinical application of this peptide. Herein, we report the synthesis of six analogues of teixobactin, in which the non-canonical L-allo-enduracididine amino acid is replaced by isosteric, commercially available Fmoc-amino acid building blocks. Biological evaluation of the analogues has revealed promising activity, particularly for guanidine isosteres, against AMR strains of S. aureus and Enterococcus faecalis, highlighting the potential for this class of cyclic depsipeptides in the treatment of Gram-positive infections.


 Please wait while we load your content...
Please wait while we load your content...