Electrophilic activation of aminocarboxylic acid by phosphate ester promotes Friedel–Crafts acylation by overcoming charge–charge repulsion†
Abstract
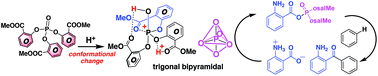
Friedel–Crafts acylation of aromatic compounds with aminocarboxylic acids proceeds efficiently in the presence of a tailored phosphate ester and a strong Brønsted acid, despite the strong charge–charge repulsion associated with acylium ion formation. Here, we investigate the mechanism of this electrophilic aromatic acylation reaction, focusing on how the aminocarboxylic acid is activated by the phosphate ester and how the charge–charge repulsion is overcome. In the first step of the reaction, an acyl phosphate is generated from the aminocarboxylic acid through the intervention of the phosphate ester, which possesses three methyl salicylate ester linkages. The o-methyl salicylates enhance the reactivity of the phosphate ester via a protonation-induced conformational change, thereby overwhelming the charge–charge repulsion associated with the acylium ion formation. Weakening of the resonance interaction in the C(O)–O(P) bond by the lone-pair electrons of the ether oxygen atom of the carboxylic acid functionality contributes to the rapid formation of the acylium ion. Thus, our results show that the formation of aromatic ketones from various carboxylic acids proceeds because the strong leaving ability of the acyl phosphate overwhelms the charge–charge repulsion associated with the formation of the acylium ion. This information will be helpful to improve the design of tailored phosphate reagents.


 Please wait while we load your content...
Please wait while we load your content...