Pseudouridine modifications influence binding of aminoglycosides to helix 69 of bacterial ribosomes†
Abstract
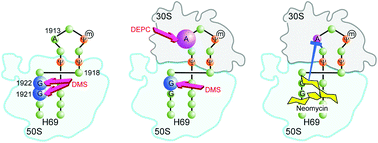
Development of antibiotics that target new regions of functionality is a possible way to overcome antibiotic resistance. In this study, the interactions of aminoglycoside antibiotics with helix 69 of the E. coli 23S rRNA in the context of complete 70S ribosomes or the isolated 50S subunit were investigated by using chemical probing and footprinting analysis. Helix 69 is a dynamic RNA motif that plays major roles in bacterial ribosome activity. Neomycin, paromomycin, and gentamicin interact with the stem region of helix 69 in complete 70S ribosomes, but have diminished binding to the isolated 50S subunit. Pseudouridine modifications in helix 69 were shown to impact the aminoglycoside interactions. These results suggest a requirement for a specific conformational state of helix 69 for efficient aminoglycoside binding, and imply that this motif may be a suitable target for mechanism-based therapeutics.
- This article is part of the themed collections: Celebrating excellence in research: women of organic chemistry and Nucleic Acid Modifications


 Please wait while we load your content...
Please wait while we load your content...