Synthesis of spirocyclic orthoesters by ‘anomalous’ rhodium(ii)-catalysed intramolecular C–H insertions†
Abstract
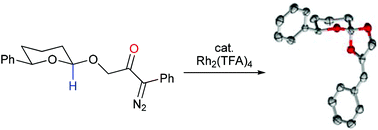
A tetrahydropyranyl acetal bearing a proximal phenyl diazoketone substituent underwent Rh(II)-catalysed C–H insertion via an ‘anomalous’ C–O bond-forming, rather than C–C bond-forming, transformation, giving spirocyclic orthoesters. Density functional theory calculations with M06 show that the formation of these anomalous products involves hydride transfer to the rhodium carbene, giving an intermediate zwitterion which undergoes C–O bond formation in preference to C–C bond formation.


 Please wait while we load your content...
Please wait while we load your content...