On the regiochemical differences between Pd-catalyzed heterocyclization–allylation and –arylation reactions of alkynylbenzamides: preparation of 4-allyl-isochromen-1-imines and computational study†
Abstract
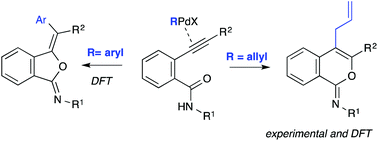
The Pd(0)-catalyzed cyclization–allylation reactions of 2-alkynylbenzamides proceed with high regioselectivity to afford the 6-endo-cyclization-derived products 4-allyl-isochromen-1-imines. DFT calculations have been performed on this and the related arylation reaction, that has been reported to afford the products corresponding to an exo-cyclization under similarly Pd(0)-catalyzed conditions. Under the reaction conditions, these cyclizations are presumed to be triggered by activation of the C–C triple bond with either an allyl- or an aryl palladium complex, generated by oxidative addition of an allyl- or aryl halide to the Pd(0) catalyst. For reactions promoted by allylpalladium species, calculations predict a reversible cyclization, followed by a regioselectivity-determining endo-selective reductive elimination. In contrast, according to calculations, the corresponding arylations would proceed through irreversible exo-selective cyclization and reductive elimination steps. These predictions are consistent with the experimental observations. The divergent regiochemical outcome appears to have its origin in the differences caused on the intermediate palladium complexes by the groups derived from the coupling agents (allyl or aryl) and by the reaction conditions (solvent and ligands) through a subtle interplay of polarity and coordinative effects.


 Please wait while we load your content...
Please wait while we load your content...