Enantioselective organocatalytic Michael additions of N,N′-dialkylbarbituric acids to enones†
Abstract
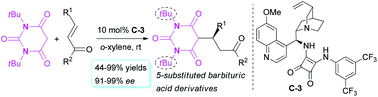
N,N′-Dialkylbarbituric acids as cyclic malonamide donors were successfully used in the enantioselective Michael addition reaction of enones. Using cinchona alkaloid-based bifunctional squaramide as an organocatalyst, this Michael reaction of N,N′-di-tert-butylbarbituric acid with various enones features a highly enantioselective (91–99% ee) production of the corresponding optically active 5-substituted barbituric acid derivatives. The transformations of the Michael product for the barbituric acid structural unit were realized in two ways, deprotection to remove the N-tert-butyl group and alkylation to produce 5,5-disubstituted barbituric acid derivatives.


 Please wait while we load your content...
Please wait while we load your content...