Synthesis of functionalized indolizines via gold(i)-catalyzed intramolecular hydroarylation/aromatization of pyrrole-ynes†
Abstract
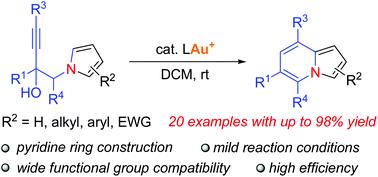
A gold-catalyzed intramolecular hydroarylation/aromatization of pyrrole-ynes has been developed. This method provides a concise and straightforward route to functionalized indolizines through the construction of the pyridine ring of indolizines and also allows elaboration of its pyrrole moiety with or without functional groups. In addition, a wide variety of functional groups, such as aryl, alkenyl, alkynyl, pyridyl or thienyl groups, can be easily incorporated into the pyridine unit of the indolizine products under mild conditions. The utility of the indolizine products was demonstrated by their efficient transformations into various C3-functionalized indolizine derivatives.
- This article is part of the themed collection: Celebrating excellence in research: women of organic chemistry


 Please wait while we load your content...
Please wait while we load your content...