Fluorescent and colorimetric molecular recognition probe for hydrogen bond acceptors†
Abstract
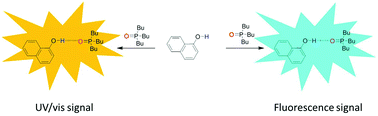
The association constants for formation of 1 : 1 complexes between a H-bond donor, 1-naphthol, and a diverse range of charged and neutral H-bond acceptors have been measured using UV/vis absorption and fluorescence emission titrations. The performance of 1-naphthol as a dual colorimetric and fluorescent molecular recognition probe for determining the H-bond acceptor (HBA) parameters of charged and neutral solutes has been investigated in three solvents. The data were employed to establish self-consistent H-bond acceptor parameters (β) for benzoate, azide, chloride, thiocyanate anions, a series of phosphine oxides, phosphate ester, sulfoxide and a tertiary amide. The results demonstrate both the transferability of H-bond parameters between different solvents and the utility of the naphthol-based dual molecular recognition probe to exploit orthogonal spectroscopic techniques to determine the HBA properties of neutral and charged solutes. The benzoate anion is the strongest HBA studied with a β parameter of 15.4, and the neutral tertiary amide is the weakest H-bond acceptor investigated with a β parameter of 8.5. The H-bond acceptor strength of the azide anion is higher than that of chloride (12.8 and 12.2 respectively), and the thiocyanate anion has a β value of 10.8 and thus is a significantly weaker H-bond acceptor than both the azide and chloride anions.
- This article is part of the themed collection: Celebrating excellence in research: women of organic chemistry


 Please wait while we load your content...
Please wait while we load your content...