Stereoselective annulation between an allene, an alkene, and two nitrosoarenes to access bis(isoxazoliodine) derivatives†
Abstract
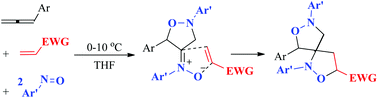
This work reports metal-free annulations between one allene, two nitrosoarenes and one electron-deficient alkene to afford bis(isoxazolidine) derivatives stereoselectively. This process involves an initial formation of isoxazolidin-4-imine oxides, followed by their dipolar [3 + 2]-cycloaddition with electron-deficient alkenes. To highlight the utility, the annulations of 5-alleneyl-1-enes with nitrosoarenes were also feasible to afford the desired bis(isoxazolidine) products with excellent stereocontrol. The resulting bis(isoxazolidine) products produced from two systems were reduced with Zn/MeOH to induce reductive N–O cleavages, yielding branched polyaminols stereoselectively.


 Please wait while we load your content...
Please wait while we load your content...