Copper-catalyzed ipso-selenation of aromatic carboxylic acids†
Abstract
The copper-catalyzed decarboxylative selenation of aromatic carboxylic acids with diselenide is reported. This transformation tolerated a diverse set of functional groups on the substrates, including pentafluorobenzoic acid and heteroaromatic acids, delivering diaryl and methyl aryl selenides in good to excellent yields. Mechanistic studies indicated that the copper catalyst is essential in the activation of the Se–Se bond and the decarboxylation of aromatic acids. The utility of the products has been demonstrated in the facile synthesis of 10H-phenoselenazine and 11-methyldibenzo-(b,f)-1,4-selenazepine.
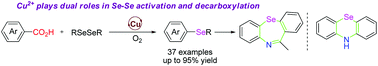


 Please wait while we load your content...
Please wait while we load your content...