NHC-catalyzed regiodivergent syntheses of difunctionalized 3-pyrazolidinones from α-bromoenal and monosubstituted hydrazine†
Abstract
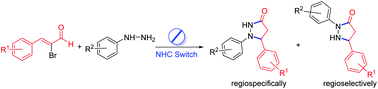
A formal [3 + 2] annulation of α-bromoenal with monosubstituted hydrazine could give 1,5 or 2,5-difunctionalized 3-pyrazolidinone regiodivergently by tuning the structure of the N-Heterocyclic Carbene (NHC) catalyst. Moderate to high yields, mild reaction conditions, good regioselectivity and potential biological significance of the final product have made this protocol attractive for the assembly of 3-pyrazolidinone.


 Please wait while we load your content...
Please wait while we load your content...