A convenient synthesis of 1-aryl-1H-1,2,3-triazoles from aliphatic substrates†
Abstract
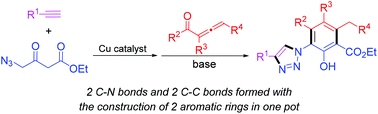
A convenient synthesis of 1-aryl-1H-1,2,3-triazoles through the one pot cascade reactions of alkynes with aliphatic azides and allenic ketones is presented. Mechanically, the formation of the title compounds involves a copper-catalyzed cycloaddition of alkyne with 4-azido-3-oxobutanoate to give 3-oxo-4-(1H-1,2,3-triazol-1-yl)butanoate as a key intermediate followed by its [3 + 3] annulation with allenic ketone through Michael addition and intramolecular condensation. A comparison study showed that using aliphatic azide as the substrate is superior to aromatic azide in terms of efficiency and selectivity. Furthermore, the 1-aryl-1H-1,2,3-triazoles bearing a 2′-bromophenyl unit attached on the 2-position of the in situ formed aryl ring obtained herein could be readily transformed into the biologically and pharmaceutically valuable tretracyclic triazolophenanthridines with good yields via a Pd-catalyzed C–H arylation.


 Please wait while we load your content...
Please wait while we load your content...