A computational investigation of the solvent-dependent enantioselective intramolecular Morita–Baylis–Hillman reaction of enones†
Abstract
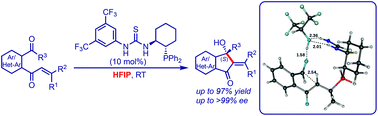
The intramolecular Morita–Baylis–Hillman reaction of β-mono and β,β-disubstituted enones shows high yields and stereo-selective products when the reaction is performed in the presence of 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) as a solvent. In this work, we carried out a computational study using the density functional Becke 3-(B3) parameter exchange and the Lee–Yang–Parr (LYP) correlation functional and 6-31+G* basis set. Two major steps i.e. C–C bond formation and proton transfer were analysed using different pathways. The calculations show a very low barrier (ΔG† = 0.23 kcal mol−1) for C–C bond formation in the case of an experimentally dominant product. For this pathway the subsequent proton transfer via the HFIP step has a barrier of 26.98 kcal mol−1. The same has been confirmed using a Molecular Electrostatic Potential (MESP), which shows a negative region in between C⋯C in the transition state. In spite of high barriers for proton transfer, the intermediate products formed in this reaction pathway are thermodynamically more stable in comparison with other pathways. The thermodynamic stability of the final product in this pathway is observed to surpass all other effects in the presence of HFIP, thereby corroborating the experimentally observed enantioselectivity.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...