One-pot synthesis of 2,3-difunctionalized indoles via Rh(iii)-catalyzed carbenoid insertion C–H activation/cyclization†
Abstract
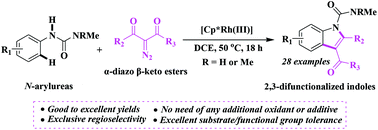
Reported herein is the first Rh(III)-catalyzed carbenoid insertion C–H activation/cyclization of N-arylureas and α-diazo β-keto esters. The redox-neutral reaction has the following features: good to excellent yields, broad substrate/functional group tolerance, exclusive regioselectivity, and no need for additional oxidants or additives, which render this methodology as a more efficient and versatile alternative to the existing methods for the synthesis of 2,3-difunctionalized indoles.


 Please wait while we load your content...
Please wait while we load your content...