The diaza-Nazarov cyclization involving a 2,3-diaza-pentadienyl cation for the synthesis of polysubstituted pyrazoles†
Abstract
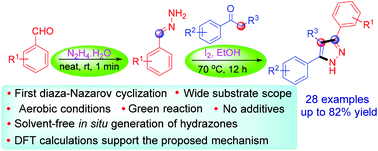
An unprecedented iodine-mediated diaza-Nazarov (DAN) type cyclization for the construction of substituted pyrazoles from easily available starting materials via an enamine–iminium ion intermediate is described. The oxidative cyclization worked under green conditions with remarkable regioselectivity. This one-pot, efficient and operationally simple three-component intramolecular regioselective DAN cyclization displayed a wide range of substrate scope. The dichotomy of reaction pathways has been explored with density functional theory in the gas phase and solution phase. Of the possible 1,5-, 1,6-, and 1,7-electrocyclizations, the DAN cyclization, i.e., the 1,5-pathway offers the lowest activation energy barrier supporting our experimental observations.


 Please wait while we load your content...
Please wait while we load your content...