Synthetic versatility of 2-substituted-6-methyl 2,3-dihydropyridinones in the synthesis of polyfunctional piperidine-based compounds and related β amino acid derivatives†
Abstract
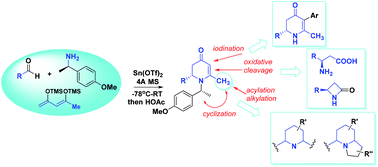
Chiral 2-substituted-6-methyl 2,3-dihydropyidinones 9, which can be facilely obtained from an asymmetric vinylogous Mannich reaction (VMR) with 1,3-bis-trimethysily enol ether, were used as versatile intermediates in constructing chiral polyfunctional piperidine-based compounds. The 6-methyl group of such compounds can be conveniently functionalized via alkylation and acylation reactions to provide efficient entries to the synthesis of a variety of chiral multi-substituted piperidine-based compounds. Further elaboration of the corresponding intermediates also provided access to polyfunctional indolizidine-based compounds. These methods were showcased in an asymmetric synthesis of 2,6-di-substituted piperidine compound 13, reported as the key intermediate in the synthesis of (+)-calvine and a natural alkaloid (−)-indolizidine 209D. Furthermore, selective C5 iodination of compound 9 enabled the installation of additional functional groups at this position. Finally, we demonstrated that the oxidative cleavage of 2-substituted-6-methyl-2,3-dihydropyidinones is a practical and efficient method for the enantioselective synthesis of β-amino acids, which can undergo further intra-molecular cyclization to give the corresponding chiral four-membered β-lactam derivatives.


 Please wait while we load your content...
Please wait while we load your content...