Palladium-catalyzed, unsymmetrical homocoupling of thiophenes via carbon–sulfur bond activation: a new avenue to homocoupling reactions†
Abstract
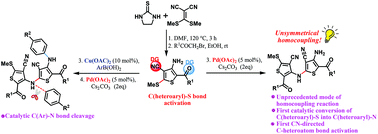
The Pd-catalyzed, CN-directed unsymmetrical synthesis of 2,4′-bithiophenes via an unprecedented homocoupling reaction is described. The NH2/CN/SMe arrangement breaks the routine. The cooperative performance of the functional groups in thiophenes would open up a new vision in the field of metal catalysis homocoupling reactions by joining the electrophilic and nucleophilic motifs of the substrate. Furthermore, it is found that the α-chelating effect of the carbonyl group in amino thiophene offers a new class of synthetic protocols for C–N cross-coupling with arylboronic acids. The bidentate N,O-chelation provides a series of advantages such as copper-catalyzed, ligand- and base-free under open-flask conditions. Interestingly, the combination of the C–N cross-coupling/homocoupling reactions in a domino fashion led to the bithiophene adducts featuring the C(Ar)–N bond cleavage in the nitrogen that bridged between the two thiophene units.


 Please wait while we load your content...
Please wait while we load your content...