Recent advances in the synthesis of analogues of phytohormones strigolactones with ring-closing metathesis as a key step†
Abstract
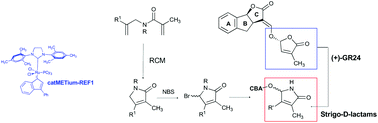
In this paper, we synthesized and evaluated the biological activity of structural analogues of natural strigolactones in which the butenolide D-ring has been replaced with a γ-lactam. The key step to obtain the α,β-unsaturated-γ-lactam was an RCM on suitably substituted amides. Strigolactones (SLs) are plant hormones with various developmental functions. As soil signaling chemicals, they are required for establishing beneficial mycorrhizal plant/fungus symbiosis. Beside these auxinic roles, recently SLs have been successfully investigated as antitumoral agents. Peculiar to the SL perception system is the enzymatic activity of the hormone receptor. SARs data have shown that the presence of the butenolide D-ring is crucial to retain the biological activity. The substitution of the butenolide with a lactam might shed light on the mechanism of perception. In the following, a dedicated in silico study suggested the binding modes of the synthesized compounds to the receptor of SLs in plants.


 Please wait while we load your content...
Please wait while we load your content...